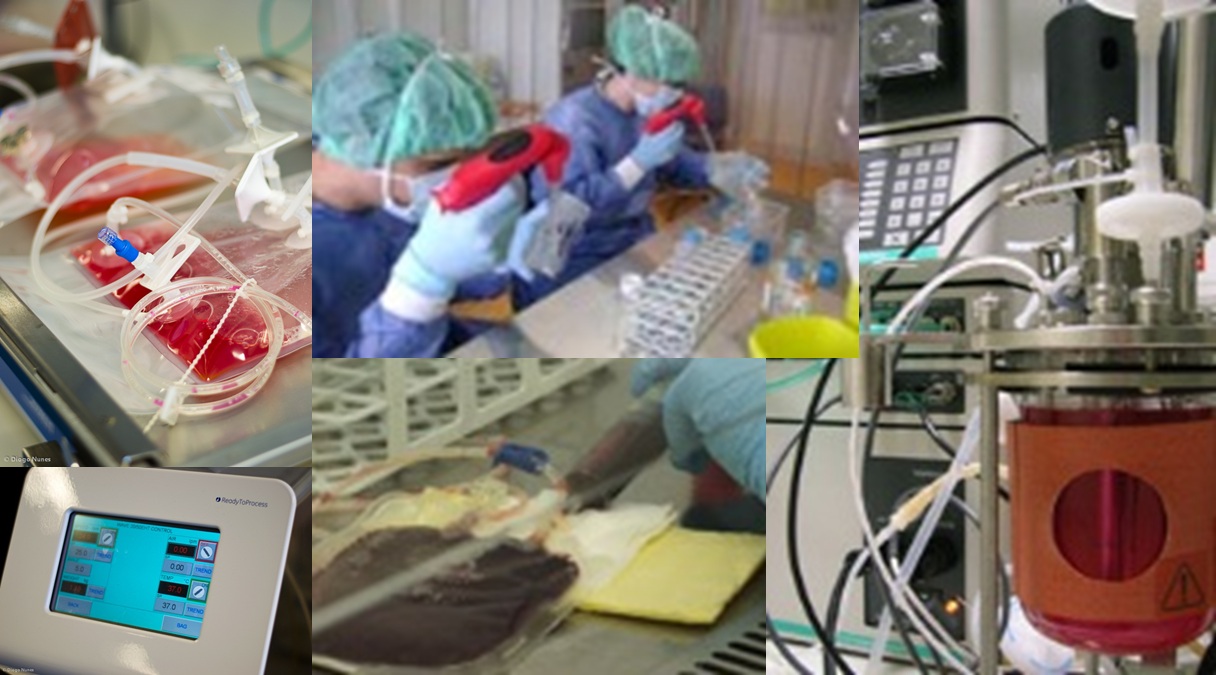
Stem Cell Bioengineering
The Stem Cell Bioengineering and Regenerative Medicine Laboratory at
IBB-BERG-IST aims at developing highly controlled bioreactor systems
for the ex-vivo expansion of stem cells and their controlled
differentiation into specific cell types, as well as their integration
with advanced bioseparation and purification techniques. As stem cells
are rare, their isolation and expansion/differentiation in vitro
significantly increases the cell population available for Cellular and
Gene therapy, Tissue Engineering, high- throughput drug screening and
stem cell research. Human hematopoietic stem cells, human mesenchymal
stem cells, as well as human and mouse pluripotent stem cells (both
embryonic stem cells and induced pluripotent stem cells) and neural
stem cells are used as model systems.
Research Projects
Ex-vivo Expansion of Adult Stem Cells
Ex-vivo expansion of HSC in co-culture with MSC under serum-free conditions - Current research is focused on: (i) the definition of optimal culture conditions namely concerning cytokine combinations, enrichment procedures and initial cell concentrations used to provide an amplification of HSC, especially those obtained from the umbilical cord blood (UCB); and (ii) the understanding of the mechanisms underlying the hematopoietic supportive capacity of MSC. These will have implications in terms of bioreactor design towards the maximization of human HSC expansion in vitro. Current research also focuses on platelet production from the ex-vivo expanded HSC. Isolation and purification methods of human hematopoietic stem/progenitor cells are being developed in collaboration with BEL, to obtain highly enriched cell populations at large-scale.
Clinical-scale production of MSC for Cellular Therapies - Culture protocols are being optimized for the isolation and expansion of MSC under serum-/xeno(geneic)-free conditions, while maintaining their multilineage differentiation and immunosuppressive capacities, as well as their genetic stability. MSC are isolated from adult bone marrow (BM), adipose tissue (AT), umbilical cord matrix (UCM) and synovial membrane. Culture of MSC in fully controlled bioreactors using microcarriers, under defined, xeno-free conditions, is currently being exploited to maximize MSC yield. In addition, a proteomic analysis platform is being established in collaboration with BSRG, IBB/CEBQ, to understand how the ex-vivo culture process affects MSC features at the proteome level.
Bioprocessing of pluripotent and neural stem cells
The ex-vivo expansion of pluripotent stem cells (PSC) and PSC-derived NSC is studied towards the definition of highly controlled bioreactor systems to establish an efficient, reproducible and cost-effective large-scale bioprocess to obtain the starting material to generate mature cells (i.e. neurons) for potential use in the treatment of neurological disorders, as well as for drug screening. Bioseparation and purification methods of human PSC-derived cells are addressed to ensure the quality control for cellular therapies.
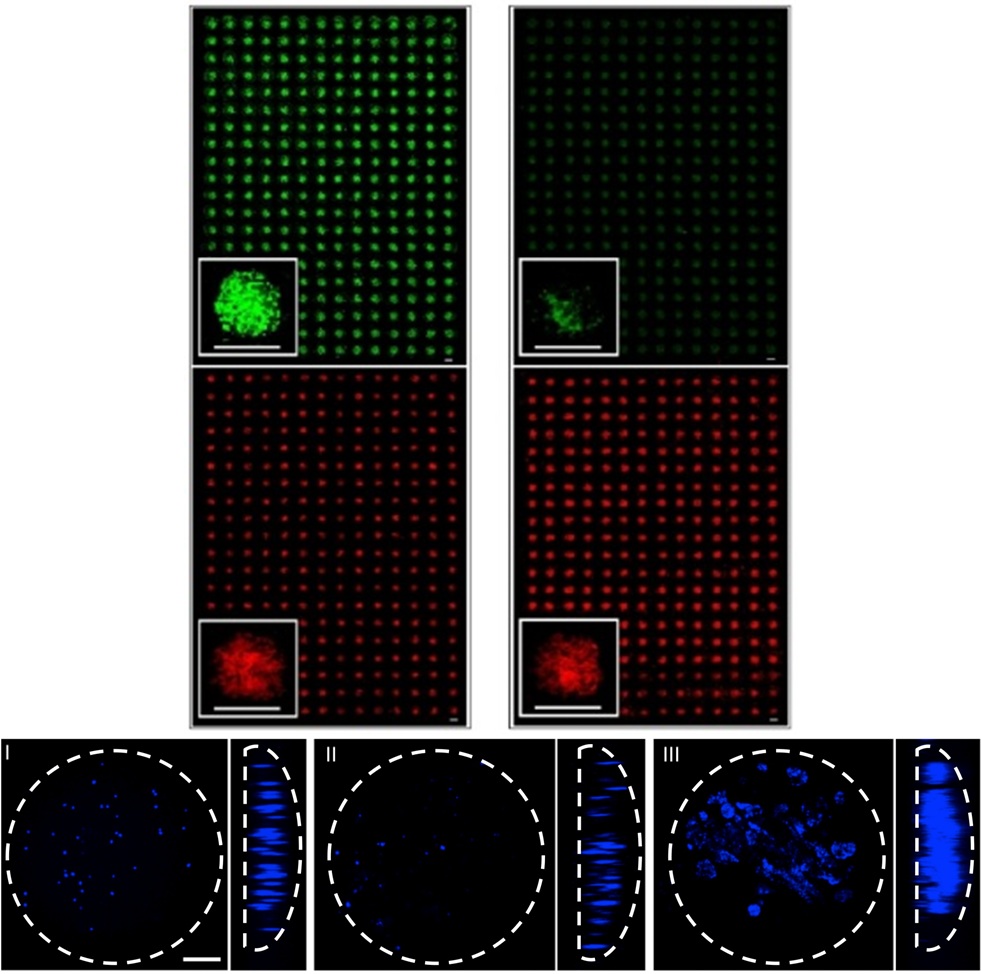
Micro-Scale culture of pluripotent stem cells
High-throughput microarray systems, as well as microfluidic devices are being developed to elucidate important microenvironmental factors (i.e. chemical, physical) affecting mouse and human pluripotent stem cell self-renewal and differentiation, while providing the basis for rapid identification of signals and conditions that can be used to direct cellular responses.
Gene delivery strategies to stem cells
Safe and effective non-viral strategies to genetically-engineer stem
cells are being developed to enhance the therapeutic efficacy in
different clinical settings. DNA vectors encoding for reporter and/or
specific proteins involved in ex-vivo expansion or differentiation of
stem cells are being delivered to these cells by microporation or
associated to cationic lipids. Novel gene carriers such as minicircles
and miniplasmids are currently being exploited, also in collaboration
with NABL, to extend gene expression and augment cell survival and
proliferation, foreseeing the maximization of stem cells for
applications in Cellular and Gene Therapy, as well as Tissue
Engineering.
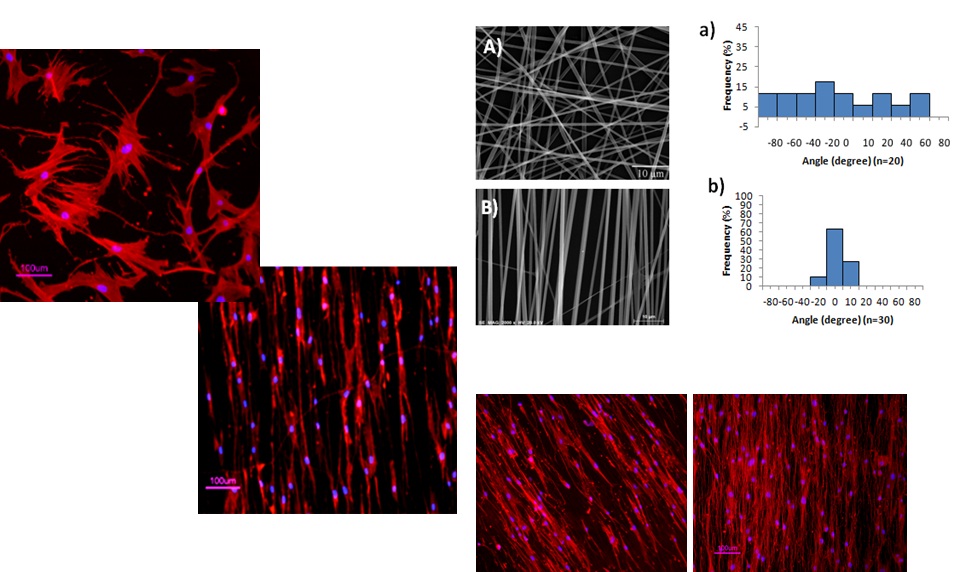
Tailoring biomaterials to support stem cell cultivation
Synthetic polymeric supports are developed to assist scalable culture
systems for maximization of ex-vivo stem cell expansion or
differentiation. Electrospinning is currently being used to produce
nanofiber scaffolds to mimic aspects of the extracellular matrix,
promoting cell-cell and cell-material interactions and cellular
adhesion. Moreover, some biomedical applications require cell recovery
from the polymeric support at the end of the cell cultivation stage.
Polymers sensitive to harmless stimuli (e.g. glucose, temperature) are
being tailored to release cells, without affecting cell viability and
function, at physiologic conditions.